T-cell delivery devices
2020 GeekWire Award Winner in the category “Health Innovation of the Year”
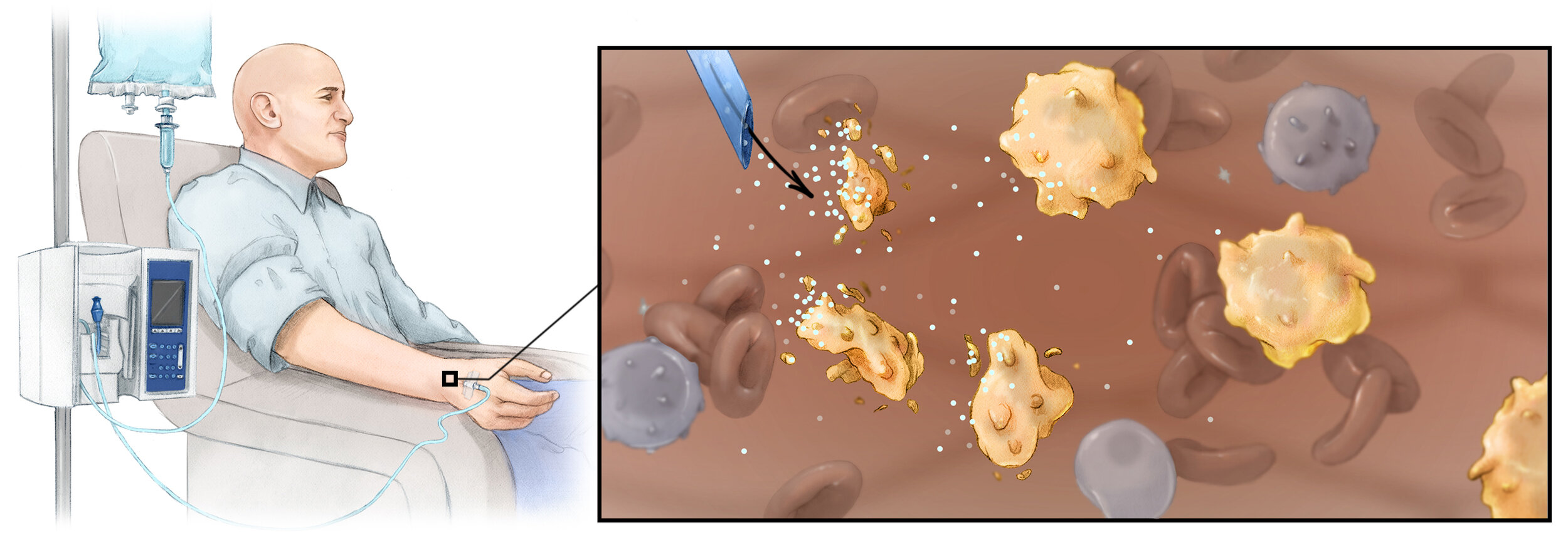
Cancer relapse after surgery is a clinical problem that often carries fatal consequences. Our group developed a bioactive polymer implant that can deliver, expand and disperse tumor-reactive T cells as a treatment for incompletely resected or inoperable tumors. These implants can be surgically situated at resection sites, or directly applied to tumors. Our interdisciplinary studies, published in Nature Biotechnology demonstrate for the first time that launching cancer-fighting immune cells from polymeric devices can safely and effectively prevent relapse and provide an effective treatment option for inoperable tumors. Based on its potential to maximize treatment success and substantially improve the survival of patients receiving tumor surgery, our technology was recently licensed for further development with the goal to maximize the success of tumor surgery, and to spare patients from repetitive operations, extended hospital stays, and rounds of radiation or chemotherapy.
The Stephan laboratory also developed a thin-film biomaterial (~10 µm-thin), that can deliver anti-cancer lymphocytes at ultra-high densities directly to the tumor. This delivery system can be incorporated into as a covering membrane for self-expandable stents used to palliate luminal gastrointestinal and endobronchial neoplasms; this provides a novel approach to minimize the risk of re-stenosis
a. Stephan SB, Taber AM, Jileaeva I, Pegues EP, Sentman CL, Stephan MT.* Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nature Biotechnology, 2015 Jan;33(1):97-101. PMCID: PMC4289408.
b. Smith TT, Moffett HF, Stephan SB, Dumigan AG, Pillarisetty VG & Stephan MT. Implants co-delivering engineered T-cells and STING agonist limit tumor immune escape. Journal of Clinical Investigation. 2017. Jun, 1;127(6):2176-2191. PMCID: PMC5451231.
c. Coon ME, Stephan SB, Gupta V, Kealey CP, Stephan MT. Nitinol thin films functionalized with CAR-T cells for the treatment of solid tumors. Nature Biomedical Engineering. 2020 Feb;4(2):195-206
2. Injectable lymphocyte-reprogramming nanocarriers
Currently, clinical-scale manufacturing of T lymphocytes is a multistep process that integrates an assortment of elaborate protocols to isolate, genetically modify, and selectively expand the redirected cells before infusing them back into the patient. Because these laborious procedures require unusual equipment and exceptional expertise, they can only be performed at a few specialized centers worldwide. Clearly, given the challenges this disease already poses to our health care system, providing personalized T- cell therapy to the more than 1.5 million new cancer patients diagnosed annually in the United States is not foreseeable. My research group developed lymphocyte-targeted nanoparticles to house genes for chimeric antigen receptors or T-cell receptors that can confer this specificity, without having to isolate lymphocytes from the patient and perform the elaborate ex vivo procedures that are used today. With a new Fred Hutch spinout company, Tidal Therapeutics , we are currently developing this platform for clinical use. Tidal Therapeutics was acquired by Sanofi in April 2021.
https://www.sanofi.com/en/media-room/press-releases/2021/2021-04-09-17-45-00-2207664
In parallel, our group has developed an off-the-shelf nanoreagent that carries in vitro transcribed (IVT) mRNA to reprogram immunosuppressive macrophages (TAMs) to express tumor-clearing phenotypes. Much effort has been devoted to developing new therapies that target TAMs as an attractive alternative to classic tumor treatment. However, the only option to date that has shown promise is systemic cytokine blockade using antibodies or small molecule drugs, but because they suppress all macrophages in the body, these treatments induce dangerous side effects. We developed a different approach: Instead of systemically ablating macrophages through cytokine inhibition we deliver genes encoding transcription factors that reprogram macrophage function within tumors. More specifically, we established that suppressive M2 macrophages can be genetically reconfigured in situ to highly effective, tumor-clearing M1 macrophages by nanoparticles targeted to provide them with genes encoding master regulators of macrophage polarization. Using three different preclinical cancer models, we demonstrated that repeated administration of these nanoreagents causes regression of cancer. Our next step is to translate this technology into the clinic as a new approach of treating otherwise incurable ovarian cancer
a. Smith TT, Stephan SB, Moffett HF, McKnight LE, Ji W, Reiman D, Bonagofski E, Wohlfahrt ME, Pillai SPS & Stephan MT. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nature Nanotechnology. 2017. Aug; 12(8):813-820. PMCID: PMC5646367.*
*Highlighted in: Scudellari M. Attack of the killer clones. Nature. 2017 Dec 21;552(7685):S64-S66.
b. Moffett HF, Coon ME, Radtke S, Stephan SB, McKnight L, Lambert A, Stoddard BL, Kiem HP, Stephan MT. Hit-and-run programming of therapeutic cytoreagents using mRNA nanocarriers. Nature Communications. 2017 Aug 30;8(1)389. PMCID: PMC5577173
c. Zhang F, Parayath NN, Ene CI, Stephan SB, Koehne AL, Coon ME, Holland EC & Stephan MT. Genetic programming of macrophages to perform anti-tumor functions. Nature Communications. 2019 Sep 3;10(1):3974. PMCID: PMC6722139
d. Parayath NN, Stephan SB, Koehne AL, Nelson PS & Stephan MT. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nature Communications. 2020 Nov 27;11(1):6080